



Liquid crystals (LCs) are a state of matter which has properties between those of conventional liquids and those of solid crystals. For instance, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of liquid-crystal phases, which can be distinguished by their different optical properties (such as textures). The contrasting areas in the textures correspond to domains where the liquid-crystal molecules are oriented in different directions. Within a domain, however, the molecules are well ordered. LC materials may not always be in a liquid-crystal phase (just as water may turn into ice or steam).
Liquid crystals can be divided into thermotropic, lyotropic and metallotropic phases. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the liquid-crystal phase as temperature is changed. Lyotropic LCs exhibit phase transitions as a function of both temperature and concentration of the liquid-crystal molecules in a solvent (typically water). Metallotropic LCs are composed of both organic and inorganic molecules; their liquid-crystal transition depends not only on temperature and concentration, but also on the inorganic-organic composition ratio.
Examples of liquid crystals can be found both in the natural world and in technological applications. Most contemporary electronic displays use liquid crystals. Lyotropic liquid-crystalline phases are abundant in living systems but can also be found in the mineral world. For example, many proteins and cell membranes are liquid crystals. Other well-known examples of liquid crystals are solutions of soap and various related detergents, as well as the tobacco mosaic virus, and some clays.
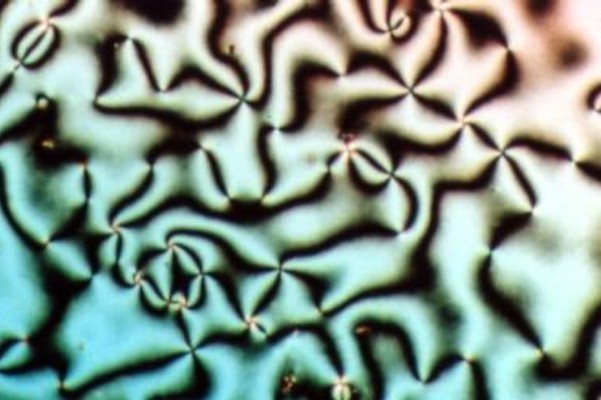
Schlieren texture of liquid crystal nematic phase
In 1888, Austrian botanical physiologist Friedrich Reinitzer, working at the Karl-Ferdinands-Universität, examined the physico-chemical properties of various derivatives of cholesterol which now belong to the class of materials known as cholesteric liquid crystals. Previously, other researchers had observed distinct color effects when cooling cholesterol derivatives just above the freezing point, but had not associated it with a new phenomenon. Reinitzer perceived that color changes in a derivative cholesteryl benzoate were not the most peculiar feature. He found that cholesteryl benzoate does not melt in the same manner as other compounds, but has two melting points. At 145.5 °C (293.9 °F) it melts into a cloudy liquid, and at 178.5 °C (353.3 °F) it melts again and the cloudy liquid becomes clear. The phenomenon is reversible. Seeking help from a physicist, on March 14, 1888, he wrote to Otto Lehmann, at that time a Privatdozent in Aachen. They exchanged letters and samples. Lehmann examined the intermediate cloudy fluid, and reported seeing crystallites. Reinitzer's Viennese colleague von Zepharovich also indicated that the intermediate "fluid" was crystalline. The exchange of letters with Lehmann ended on April 24, with many questions unanswered. Reinitzer presented his results, with credits to Lehmann and von Zepharovich, at a meeting of the Vienna Chemical Society on May 3, 1888.
By that time, Reinitzer had discovered and described three important features of cholesteric liquid crystals (the name coined by Otto Lehmann in 1904): the existence of two melting points, the reflection of circularly polarized light, and the ability to rotate the polarization direction of light.
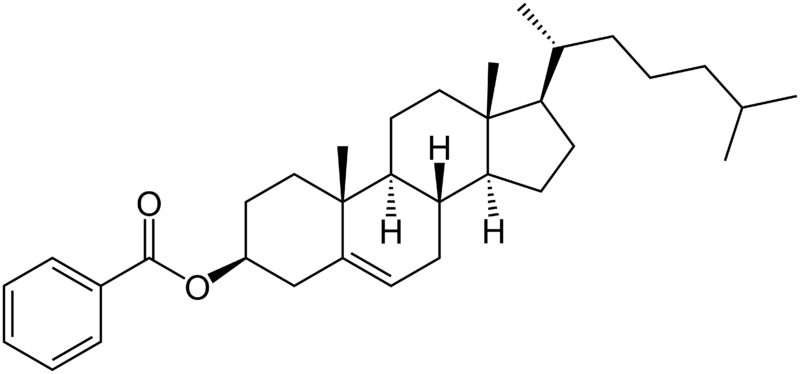
Chemical structure of cholesteryl benzoate molecule.
By that time, Reinitzer had discovered and described three important features of cholesteric liquid crystals (the name coined by Otto Lehmann in 1904): the existence of two melting points, the reflection of circularly polarized light, and the ability to rotate the polarization direction of light.
After his accidental discovery, Reinitzer did not pursue studying liquid crystals further. The research was continued by Lehmann, who realized that he had encountered a new phenomenon and was in a position to investigate it: In his postdoctoral years he had acquired expertise in crystallography and microscopy. Lehmann started a systematic study, first of cholesteryl benzoate, and then of related compounds which exhibited the double-melting phenomenon. He was able to make observations in polarized light, and his microscope was equipped with a hot stage (sample holder equipped with a heater) enabling high temperature observations.
After World War II work on the synthesis of liquid crystals was restarted at university research laboratories in Europe. George William Gray, a prominent researcher of liquid crystals, began investigating these materials in England in the late 1940s. His group synthesized many new materials that exhibited the liquid crystalline state and developed a better understanding of how to design molecules that exhibit the state. His book Molecular Structure and the Properties of Liquid Crystals became a guidebook on the subject. One of the first U.S. chemists to study liquid crystals was Glenn H. Brown, starting in 1953 at the University of Cincinnati and later at Kent State University. In 1965, he organized the first international conference on liquid crystals, in Kent, Ohio, with about 100 of the world’s top liquid crystal scientists in attendance. This conference marked the beginning of a worldwide effort to perform research in this field, which soon led to the development of practical applications for these unique materials.

George William Gray.
Liquid crystal materials became a focus of research in the development of flat panel electronic displays beginning in 1962 at RCA Laboratories. When physical chemist Richard Williams applied an electric field to a thin layer of a nematic liquid crystal at 125 °C, he observed the formation of a regular pattern that he called domains (now known as Williams Domains). This led his colleague George H. Heilmeier to perform research on a liquid crystal-based flat panel display to replace the cathode ray vacuum tube used in televisions. But the para-Azoxyanisole that Williams and Heilmeier used exhibits the nematic liquid crystal state only above 116 °C, which made it impractical to use in a commercial display product. A material that could be operated at room temperature was clearly needed.
In 1969, Hans Kelker succeeded in synthesizing a substance that had a nematic phase at room temperature, MBBA, which is one of the most popular subjects of liquid crystal research. The next step to commercialization of liquid-crystal displays was the synthesis of further chemically stable substances (cyanobiphenyls) with low melting temperatures by George Gray. That work with Ken Harrison and the UK MOD (RRE Malvern), in 1973, led to design of new materials resulting in rapid adoption of small area LCDs within electronic products.
These molecules are rod-shaped, some created in the lab and some appearing spontaneously in nature. Since then, two new types of LC molecules have been discovered, both man-made: disc-shaped (created by S. Chandrasekhar's group in India, 1977) and bowl-shaped (invented by Lui Lam in China, 1982, and synthesized in Europe three years later).
In 1991, when liquid crystal displays were already well established, Pierre-Gilles de Gennes working at the Université Paris-Sud received the Nobel Prize in physics "for discovering that methods developed for studying order phenomena in simple systems can be generalized to more complex forms of matter, in particular to liquid crystals and polymers".
A large number of chemical compounds are known to exhibit one or several liquid crystalline phases. Despite significant differences in chemical composition, these molecules have some common features in chemical and physical properties. There are three types of thermotropic liquid crystals: discotics, bowlics and rod-shaped molecules. Discotics are flat disc-like molecules consisting of a core of adjacent aromatic rings; the core in a bowlic is not flat but like a rice bowl (a three-dimensional object). This allows for two dimensional columnar ordering, for both discotics and bowlics. Rod-shaped molecules have an elongated, anisotropic geometry which allows for preferential alignment along one spatial direction.
An extended, structurally rigid, highly anisotropic shape seems to be the main criterion for liquid crystalline behavior, and as a result many liquid crystalline materials are based on benzene rings.
The various liquid-crystal phases (called mesophases) can be characterized by the type of ordering. One can distinguish positional order (whether molecules are arranged in any sort of ordered lattice) and orientational order (whether molecules are mostly pointing in the same direction), and moreover order can be either short-range (only between molecules close to each other) or long-range (extending to larger, sometimes macroscopic, dimensions). Most thermotropic LCs will have an isotropic phase at high temperature. That is that heating will eventually drive them into a conventional liquid phase characterized by random and isotropic molecular ordering (little to no long-range order), and fluid-like flow behavior. Under other conditions (for instance, lower temperature), a LC might inhabit one or more phases with significant anisotropic orientational structure and short-range orientational order while still having an ability to flow.
The ordering of liquid crystalline phases is extensive on the molecular scale. This order extends up to the entire domain size, which may be on the order of micrometers, but usually does not extend to the macroscopic scale as often occurs in classical crystalline solids. However some techniques, such as the use of boundaries or an applied electric field, can be used to enforce a single ordered domain in a macroscopic liquid crystal sample. The ordering in a liquid crystal might extend along only one dimension, with the material being essentially disordered in the other two directions.
Microscopic theoretical treatment of fluid phases can become quite complicated, owing to the high material density, meaning that strong interactions, hard-core repulsions, and many-body correlations cannot be ignored. In the case of liquid crystals, anisotropy in all of these interactions further complicates analysis. There are a number of fairly simple theories, however, that can at least predict the general behavior of the phase transitions in liquid crystal systems.
As we already saw above, the nematic liquid crystals are composed of rod-like molecules with the long axes of neighboring molecules aligned approximately to one another. To describe this anisotropic structure, a dimensionless unit vector n called the director, is introduced to represent the direction of preferred orientation of molecules in the neighborhood of any point. Because there is no physical polarity along the director axis, n and -n are fully equivalent.
The local nematic director, which is also the local optical axis, is given by the spatial and temporal average of the long molecular axes.
The description of liquid crystals involves an analysis of order. A second rank symmetric traceless tensor order parameter is used to describe the orientational order of a nematic liquid crystal, although a scalar order parameter is usually sufficient to describe uniaxial nematic liquid crystals. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
where θ is the angle between the liquid-crystal molecular axis and the local director (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its local optical axis). The brackets denote both a temporal and spatial average. This definition is convenient, since for a completely random and isotropic sample, S = 0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, S is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways; for instance, diamagnetism, birefringence, Raman scattering, NMR and EPR can be used to determine S.
The order of a liquid crystal could also be characterized by using other even Legendre polynomials (all the odd polynomials average to zero since the director can point in either of two antiparallel directions). These higher-order averages are more difficult to measure, but can yield additional information about molecular ordering.
A positional order parameter is also used to describe the ordering of a liquid crystal. It is characterized by the variation of the density of the center of mass of the liquid crystal molecules along a given vector. In the case of positional variation along the z-axis the density ρ(z) is often given by:
The complex positional order parameter is defined as Ψ(r) = ρ1(r)eiΦ(r) and ρ0 the average density. Typically only the first two terms are kept and higher order terms are ignored since most phases can be described adequately using sinusoidal functions. For a perfect nematic Ψ = 0 and for a smectic phase Ψ will take on complex values. The complex nature of this order parameter allows for many parallels between nematic to smectic phase transitions and conductor to superconductor transitions.
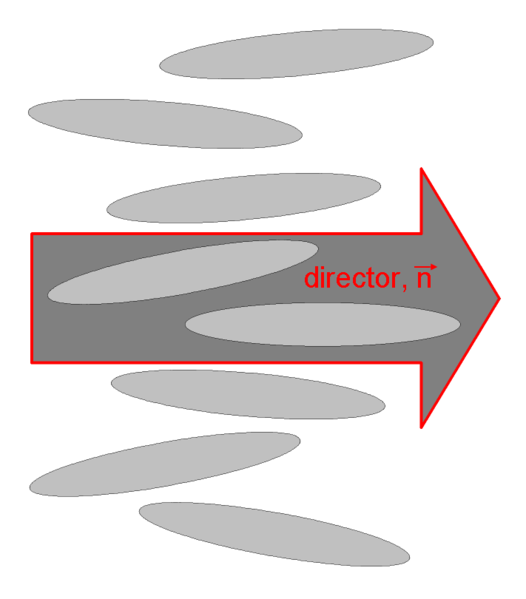
The local nematic director, which is also the local optical axis, is given by the spatial and tempor...
Liquid crystals find wide use in liquid crystal displays, which rely on the optical properties of certain liquid crystalline substances in the presence or absence of an electric field. In a typical device, a liquid crystal layer (typically 4 μm thick) sits between two polarizers that are crossed (oriented at 90° to one another). The liquid crystal alignment is chosen so that its relaxed phase is a twisted one (see Twisted nematic field effect). This twisted phase reorients light that has passed through the first polarizer, allowing its transmission through the second polarizer (and reflected back to the observer if a reflector is provided). The device thus appears transparent. When an electric field is applied to the LC layer, the long molecular axes tend to align parallel to the electric field thus gradually untwisting in the center of the liquid crystal layer. In this state, the LC molecules do not reorient light, so the light polarized at the first polarizer is absorbed at the second polarizer, and the device loses transparency with increasing voltage. In this way, the electric field can be used to make a pixel switch between transparent or opaque on command. Color LCD systems use the same technique, with color filters used to generate red, green, and blue pixels. Chiral smectic liquid crystals are used in ferroelectric LCDs which are fast-switching binary light modulators. Similar principles can be used to make other liquid crystal based optical devices. Liquid crystal tunable filters are used as electrooptical devices, e.g., in hyperspectral imaging.
Thermotropic chiral LCs whose pitch varies strongly with temperature can be used as crude liquid crystal thermometers, since the color of the material will change as the pitch is changed. Liquid crystal color transitions are used on many aquarium and pool thermometers as well as on thermometers for infants or baths. Other liquid crystal materials change color when stretched or stressed. Thus, liquid crystal sheets are often used in industry to look for hot spots, map heat flow, measure stress distribution patterns, and so on. Liquid crystal in fluid form is used to detect electrically generated hot spots for failure analysis in the semiconductor industry.

Structure of liquid crystal display: 1 – vertical polarization filter, 2,4 – glass with electrodes,...
Liquid crystal lenses converge or diverge the incident light by adjusting the refractive index of liquid crystal layer with applied voltage or temperature. Generally, the liquid crystal lenses generate a parabolic refractive index distribution by arranging molecular orientations. Therefore, a plane wave is reshaped into a parabolic wavefront by a liquid crystal lens. The focal length of liquid crystal lenses could be continuously tunable when the external electric field can be properly tuned. Liquid crystal lenses are a kind of adaptive optics. Imaging system can be benefited with focusing correction, image plane adjustment, or changing the range of depth-of-field or depth of focus. Liquid crystal lens is one of the candidates to develop vision correction device for myopia and presbyopia eyes (e.g., tunable eyeglass and smart contact lenses).
Liquid crystal lasers use a liquid crystal in the lasing medium as a distributed feedback mechanism instead of external mirrors. Emission at a photonic bandgap created by the periodic dielectric structure of the liquid crystal gives a low-threshold high-output device with stable monochromatic emission.
Polymer dispersed liquid crystal (PDLC) sheets and rolls are available as adhesive backed Smart film which can be applied to windows and electrically switched between transparent and opaque to provide privacy. Many common fluids, such as soapy water, are in fact liquid crystals. Soap forms a variety of LC phases depending on its concentration in water. Bowlic columns could be used for fast switches.
REFERENCES
Topological Microfluidics -Springer. Available in: https://www.springer.com/cda/content/document/cda_downloaddocument/9783319008578-c2.pdf?SGWID=0-0-45-1418012-p175260985. Access in: 20/10/2018.
Wikipedia. Available in: https://en.wikipedia.org/wiki/Liquid_crystal. Access in: 20/10/2018.
Wikipedia. Available in: https://en.wikipedia.org/wiki/Liquid-crystal_display. Access in: 20/10/2018.

Default timespace