



The nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell model was proposed by Dmitry Ivanenko(together with E. Gapon) in 1932. The model was developed in 1949 following independent work by several physicists, most notably Eugene Paul Wigner, Maria Goeppert Mayer and J. Hans D. Jensen, who shared the 1963 Nobel Prize in Physics for their contributions.
.
The shell model is partly analogous to the atomic shell model which describes the arrangement of electrons in an atom, in that a filled shell results in greater stability. When adding nucleons (protons or neutrons) to a nucleus, there are certain points where the binding energy of the next nucleon is significantly less than the last one. This observation, that there are certain magic numbers of nucleons:
which are more tightly bound than the next higher number, is the origin of the shell model.
The shells for protons and for neutrons are independent of each other. Therefore, one can have "magic nuclei" where one nucleon type or the other is at a magic number, and "doubly magic nuclei", where both are. Due to some variations in orbital filling, the upper magic numbers are 126 and, speculatively, 184 for neutrons but only 114 for protons, playing a role in the search for the so-called island of stability. Some semi-magic numbers have been found, notably Z=40 giving nuclear shell filling for the various elements; 16 may also be a magic number.
In order to get these numbers, the nuclear shell model starts from an average potential with a shape something between the square well and the harmonic oscillator. To this potential a spin orbit term is added. Even so, the total perturbation does not coincide with experiment, and an empirical spin orbit coupling must be added with at least two or three different values of its coupling constant, depending on the nuclei being studied.
Further evidence for such magic numbers is provided by the very high binding energy of nuclei with both Z and N being magic, and the abnormally high or low alpha and beta particle energies emitted by radioactive nuclei according to whether the daughter or parent nucleus has a magic number of neutrons. Similarly, nuclides with a magic number of neutrons are observed to have a relatively low probability of absorbing an extra neutron. To explain such nuclear systematics and to provide some insight into the internal structure of the nucleus, a shell model of the nucleus has been developed. This model uses Shrodinger's wave equation or quantum mechanics to describe the energetics of the nucleons in a nucleus in a manner analogous to that used to describe the discrete energy states of electrons about a nucleus. This model assumes:
When the model's quantum-mechanical wave equation is solved numerically (not an easy task), the nucleons are found to distribute themselves into a number of energy levels. There is a set of energy levels for protons and an independent set of levels for neutrons. Such closed shells are analogous to the closed shells of orbital electrons. However, to obtain such results that predict the magic numbers, it is necessary to make a number of ad hoc assumptions about the angular momenta of the energy states of nuclei.
Nevertheless, the magic numbers of nucleons, as well as other properties, can be arrived at by approximating the model with a three-dimensional harmonic oscillator plus a spin-orbit interaction. The model has been particularly useful in predicting several properties of the nucleus, including (1) the total angular momentum of a nucleus, (2) characteristics of isomeric transitions, which are governed by large changes in nuclear angular momentum, (3) the characteristics of beta decay and gamma decay, and (4) the magnetic moments of nuclei.
In the following figure, the single-particle levels in both the models, the nuclear shell model and the atomic shell model, are compared.
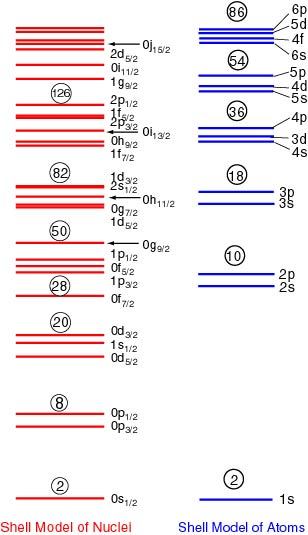
The Energy Levels in the Nuclear and Atomic Shell Models.
The red levels show the single-particle levels in the Mayer-Jensen shell model for nuclei and the blue levels are those in the atomic shell model. In each of the red levels, specified numbers of protons and neutrons are filled one by one from the bottom, and in blue levels electrons are filled. The circled numbers indicate the particle number which can be filled up to the levels. They are just the magic numbers.
REFERENCES
SHULTIS, J. Kenneth; FAW, Richard E. FUNDAMENTALS OF NUCLEAR SCIENCE AND ENGINEERING. Marcel Dekker, Inc., New York, 2002. p. 80.
Kyushu University Graduate School of Science. Available in: http://ne.phys.kyushu-u.ac.jp/seminar/MicroWorld3_E/3Part2_E/3P26_E/shell_model_E.htm. Access in: 04/10/2018.
Wikipedia. Available in: https://en.wikipedia.org/wiki/Nuclear_shell_model. Access in: 04/10/2018.

Default timespace