



.
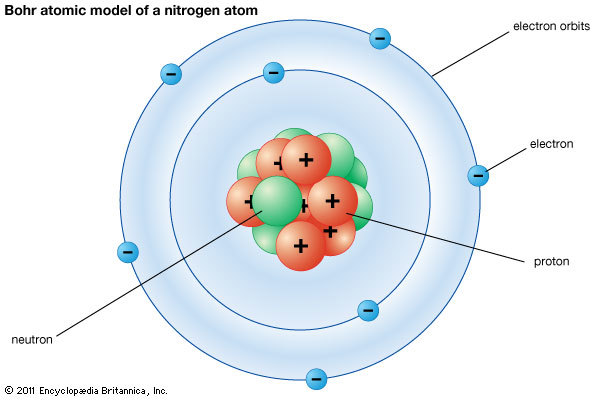
The very first-time quantum theory was incorporated in Bohr’s Model of an atom or Bohr atomic model. Later this model became the predecessor of complete quantum mechanical models. A Danish physicist named Neils Bohr in 1913 proposed the Bohr atomic model. He modified the problems and limitations associated with Rutherford’s model of an atom. The electrons move around in a predictable path called orbits. Bohr modified Rutherford’s model where he explained that electrons move around in fixed orbital shells. Furthermore, he explained that each orbital shell has fixed energy levels. According to Bohr Atomic model, a small positively charged nucleus is surrounded by revolving negatively charged electrons in fixed orbits. He concluded that electron will have more energy if it is located away from the nucleus whereas the electrons will have less energy if it located near the nucleus. This is an atomic model called semi-classical.
By limiting the orbiting electrons to a series of circular orbits having discrete radii, Bohr could account for the series of discrete wavelengths in the emission spectrum of hydrogen. Light, he proposed, radiated from hydrogen atoms only when an electron made a transition from an outer orbit to one closer to the nucleus. The energy lost by the electron in the abrupt transition is precisely the same as the energy of the quantum of emitted light. In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy. The inset shows an electron jumping from orbit n=3 to orbit n=2, emitting a photon of red light with an energy of 1.89 eV.

.
Bohr's postulates to the atomsuggested that electrons could only have certain classical motions:
Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency ν determined by the energy difference of the levels according to the Planck relation:
∆E = E2 - E1 = hf
where h is Planck's constant. The frequency of the radiation emitted at an orbit of period T is as it would be in classical mechanics; it is the reciprocal of the classical orbit period:
f = 1/T
The angular momentum, L, of the orbiting electron is quantised such that:
mevr = nħ
where me is the mass of electron, v is the velocity and n = 1, 2, 3, ... is called the principal quantum number, and ħ = h/2π. The lowest value of n is 1; this gives a smallest possible orbital radius r of 0.0529 nm known as the Bohr radius. Once an electron is in this lowest orbit, it can get no closer to the proton. Starting from the angular momentum quantum rule, Bohr was able to calculate the energies of the allowed orbits of the hydrogen atom and other hydrogen-like atoms and ions.
REFERENCES
Encyclopædia Britannica. Available in: https://www.britannica.com/science/Bohr-atomic-model. Access in: 03/09/2018.
Wikipedia. Available in: https://en.wikipedia.org/wiki/Bohr_model. Access in: 03/09/2018.

Default timespace